Abstract 22
Report on the side effects of neurophysiological evaluation with TMS in clinical trials.
Fonseca Junior, Paulo Roberto 1; Gomes, Maria Helena Sousa 1; Marchiore, Patrícia Monteiro 1; Battistella, Linamara Rizzo 1; Fregni Felipe 2; Simis, Marcel 1
- Physical and Rehabilitation Medicine Institute, General Hospital, Medical School of the University of Sao Paulo, Sao Paulo, Brazil; 2. Neuromodulation Center and Center for Clinical Research Learning, Spaulding Rehabilitation Hospital and Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA
OBJECTIVE: To describe and compare the side effects of neurophysiological evaluation with TMS of healthy participants and amputees participants with phantom limb pain.
METHODS: 112 participants were included in two trials (39 healthy participants and 73 individuals with phantom limb pain (PLP) - CAAE 76850117.3.0000.0068/ 86832518.7.0000.0068). Neurophysiological evaluations using TMS were performed measuring the motor threshold (MT), motor evoked potential (MEP), short-interval intracortical inhibition (SICI), Intracortical Facilitation (ICF), and performing cortical mapping (CM). The CM was only performed in the subjects participating in the PLP trial. This led to a total evaluation time that was twice as long compared to the duration of the evaluation of healthy participants. An adverse effect questionnaire was conducted after the TMS evaluation, including a binary question regarding the presence or absence of common side effects (headache, neck or scalp pain, and sleepiness).
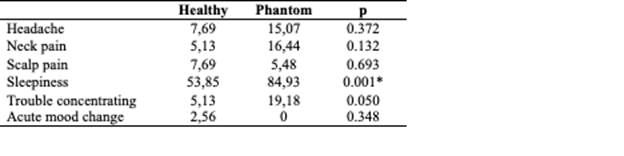
RESULTS: The presence of the adverse effects in percentages and the comparison between the groups are described in table 1. There were only minor side effects which were commonly described in previous studies. There are no differences between the groups regarding most of the side effects, with an exception for sleepiness.
CONCLUSIONS: The rate of side effects is low, with the exception of sleepiness. The higher percentage of sleepiness in amputees are likely to be related to a longer duration of evaluation, and is unlikely to be related to the TMS effect. Future studies with a placebo evaluation are necessary to confirm this hypothesis.
TABLE. Comparison of symptom results based on groups % (Fisher's exact test). Asterisk represents the p<0.05.
KEYWORDS: transcranial magnetic stimulation, TMS, side effects
ACKNOWLEDGMENTS: I would like to thank everyone from our team, such as Karin, Artur, Karinna, Natalia, Christiane, and Simon.
FUNDING/FINANCIAL SUPPORT: This work is supported by an NIH grant (R01-HD082302-01A1) and FAPESP – SPEC (2017/12943-8)
DOI: https://doi.org/10.21801/ppcrj.2020.S1.22
